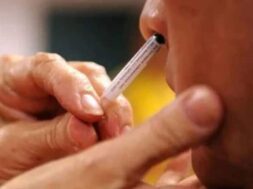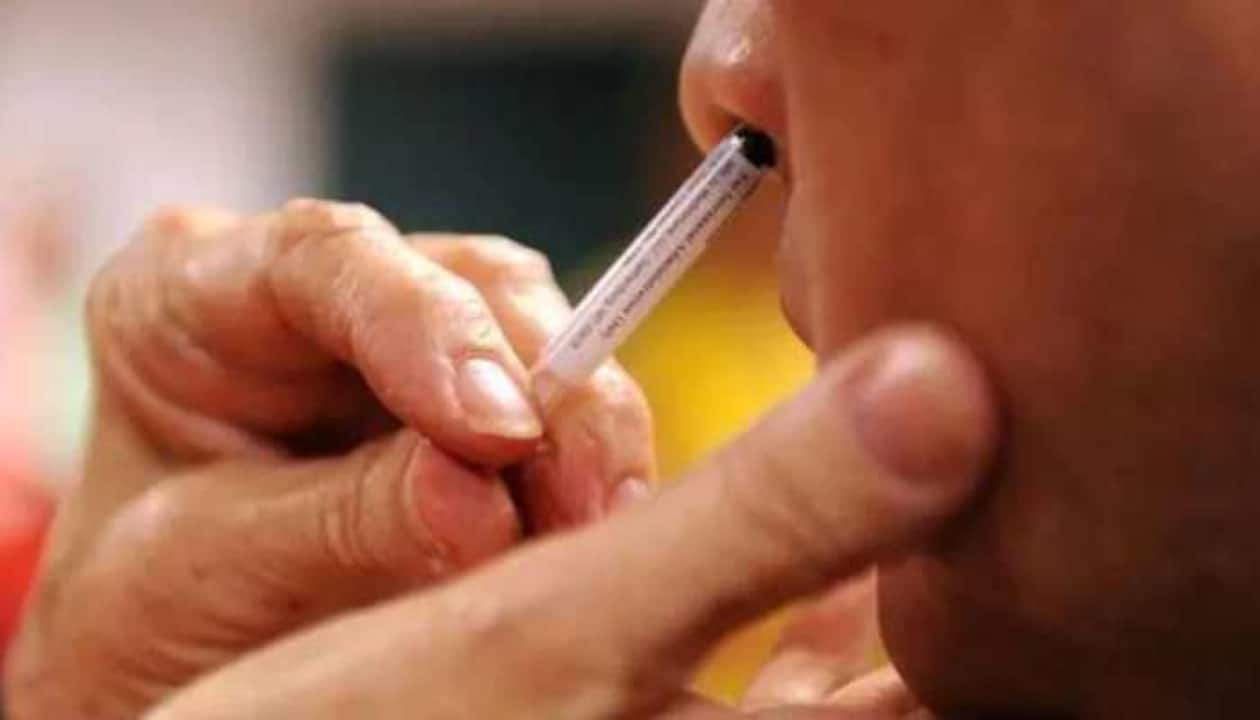
Nasal Vaccine Approved for Covid-19
NEW DELHI, Sept 6: Bharat Biotech’s nasal vaccine against Covid-19 has been approved by the Drugs Controller General of India for “restricted use” among adults “in emergency situation,” Union Health Minister Mansukh Mandaviya announced on Tuesday.
The minister said the nasal vaccine could be used for primary immunisation of those aged 18 years and above in emergency situations. This would mean that for protecting people against Coronavirus, a pathogen that invades the airway, a strategy in addition to the hitherto intramuscular shot is also available.
In February, in what was the country’s first such anti-Covid drug, Mumbai-based Glenmark launched a nasal spray (branded FabiSpray) in partnership with SaNOtize, for treatment of adult patients.
The company got manufacturing and marketing approvals from the Drugs Controller General of India for its Nitric Oxide Nasal Spray as part of an accelerated approval process. “Phase 3 trial in India met the key endpoints and demonstrated reduction of viral load of 94 per cent in 24 hours and 99 per cent in 48 hours,” the official statement said.
The vaccines delivered through nasal or oral routes help to overcome the potential difficulties with mass vaccination and reduce the cost by doing away with the need for needles and syringes. Intranasal vaccines are also expected to cut down on the dependence on various trained personnel to administer the vaccine, according to experts.
“One attraction with the intranasal vaccine is that it’s very simple to use — you just squirt it into your nose — and it’s something that can be self-administered in pandemics and outbreaks,” some experts said. Some others, like vaccine scientist Dr Gagandeep Kang, however, believe that a nasal vaccine has a likelihood of lower safety events as “it is going into a mucosal surface, it will likely be restricted.”
However, according to experts, there is very little evidence to back the effectiveness of this route of delivery so far and, save for some flu vaccines, attempts to deliver vaccines like this have not been successful.
As for the pandemic status on Tuesday, India saw a single-day rise of 4,417 coronavirus infections, which is the lowest in three months, as per government data updated at 8 am.
Active Covid cases have declined to 52,336, while there were 23 fatalities reported. The active cases now comprise 0.12 per cent of the total infections while the recovery rate has increased to 98.69 per cent, the Health Ministry said.
Vaccine development is also seeing a boost too, across the world. UK health authorities on Saturday approved a second “bivalent” vaccine to be used as a booster to target both the Omicron and original strains of the virus.
(Manas Dasgupta)