Manas Dasgupta
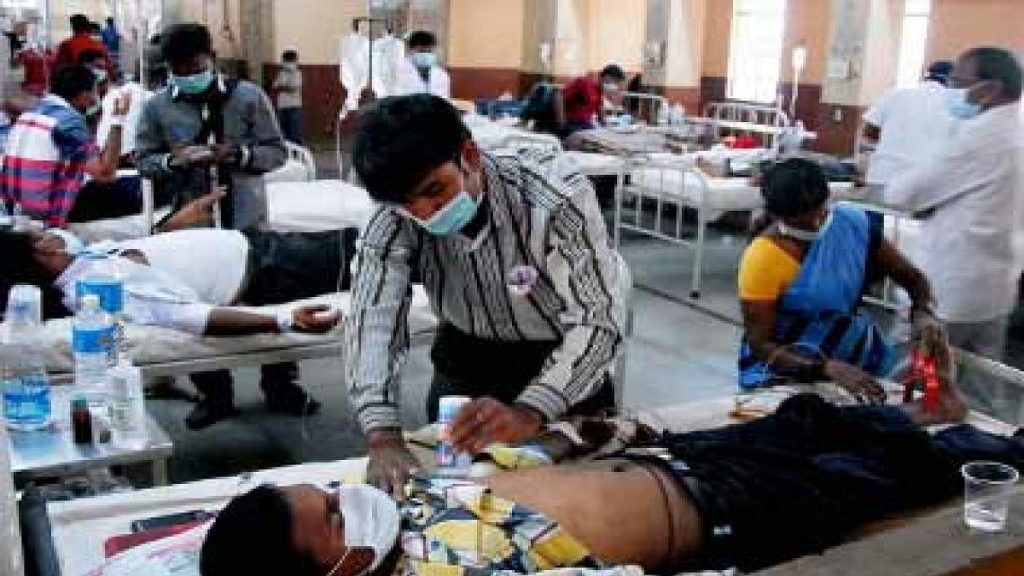
NEW DELHI, Apr 16: In a bid to mop up all available resources for the treatment of the Coronavirus patients, the union health ministry has requested all the central ministries and the public sector undertakings to make available for general Covid patients the hospital facilities with them in different parts of the country.
The ministries and the PSUs have also been advised to provide the details of the dedicated hospital facilities to the general public to avail of the advantage.
The central government said it had also decided to increase the production of the only indigenously-developed vaccine “Covaxin” to about 10 crore doses per month in the next five months and to increase the production of Remdesivir injections, widely used for the treatment of the Covid patients, in the country.
The union health ministry in a circular issued on Friday
said, “To substantially augment the hospital infrastructure for effective clinical management of severe COVID-19 patients across the country, all the Central Ministries are requested to issue instructions to the hospitals under their control or their PSUs to set-up exclusive dedicated hospital wards or separate blocks within the hospitals for COVID Care, as was done last year.”
Official sources pointed out that among all the central ministries, railways, labour and defence ministries have a large number of hospitals available at their disposal at different parts of the country.
The circular added that these hospitals/blocks should have separate entry and exit points to provide treatment services including specialised care for the confirmed COVID-19 cases.
Additionally, these dedicated hospital wards or blocks have to be equipped to provide all supportive and ancillary services including oxygen supported beds, ICU beds, ventilators and specialised critical care units (wherever available), laboratory services, imaging services, kitchen, laundry etc., along with dedicated health work force.
In his letter written to Central ministries, Health Secretary Rajesh Bhushan said the sudden surge in cases across the country calls for similar supportive action as last year from all such Ministries / Departments and their PSUs and the Hospitals under their control.
The Central Ministries have been also been advised to provide details of such dedicated hospital wards/blocks, duly coordinating with the respective health departments of States/UTs and the District Health Administration of the States/Districts wherever these hospitals are located.
It has also been suggested that a Nodal Officer may be nominated from the Ministry/Department for necessary coordination with the respective States/UTs for this purpose and their contact details be shared with the respective States/UTs as well as the Health Ministry.
Meanwhile, the union ministry of science and technology said that the current production capacity of Covaxin, which is India’s first indigenous shot against Covid-19, which was about one crore doses per month, will be doubled by May-June, then increased nearly six-seven fold by July-August and, finally, to nearly 10 crore doses per month by September.
“Under Mission COVID Suraksha, the Department of biotechnology is providing financial support as grant to vaccine manufacturing facilities for enhanced production capacities,” the ministry said in a press release.
On Bharat Biotech International Limited, which has developed the shot, the release noted that as part of the augmentation plan, the production capacities of the Hyderabad-based firm, as well as those of other public sector manufacturers, were being upgraded with required infrastructure and technology. “Financial support is being provided by the Government of India to the tune of approximately ₹65 crore to Bharat Biotech’s new Bengaluru facility, which is being repurposed to increase the capacity of vaccine production,” it added.
The ministry further mentioned that three public sector companies are also being supported to increase the vaccine production capacity: Haffkine Biopharmaceutical Corporation Limited, Mumbai; Indian Immunologicals Limited (IIL), Hyderabad; and Bharat Immunologicals and Biologicals Limited (BIBCOL), Bulandshahr.
Haffkine requested for 12 months to make its facility available for vaccine production but was asked by the government to do so “urgently” within six months, the release said, adding that once functional, the facility will be able to produce two crore doses in a month. IIL and BIBCOL, it said, will be supported to prepare their facilities to produce one-1.5 crore vaccine doses per month.
Covaxin with 81 per cent efficacy along with “Covishield” being manufactured by the Serum Institute of India are being used in the nation’s vaccination drive against the viral disease, which began on January 16, and is currently in its third phase. A third, the Russian-made Sputnik V, has just been approved for emergency use by the Drugs Controller General of India (DCGI).
The Chemicals and Fertilisers Minister D V Sadananda Gowda said on Friday that a total of 6.69 lakh vials of Remdesivir drug have been made available to different states and union territories during the last five days. The government is also taking all steps to accelerate the production of the drug, he said.
“The Government is taking every necessary step to accelerate the production facilities of #Remdesivir, it’s capacity enhancement & availability”, Gowda said in a tweet. In another tweet Gowda said: “On Govt’s intervention, major Manufacturers of #Remdesivir have voluntarily reduced its MRP ranging from ₹5,400 to less than ₹3,500 by 15.04.2021.This will support PM @narendramodi’s efforts to fight #COVID19”.
Meanwhile, a state minister of Maharashtra on Friday flagged that the state will face a shortage of 12,000 to 15,000 remdesivir injections.
Maharashtra’s Food and Drug Administration (FDA) Minister Rajendra Shingane said, “Companies that produce Remdesivir injection have increased their production but it will take some time for the vials to hit the market. If we consider a 10-12 per cent shortage, Maharashtra will continue to face a shortage of 12,000 to 15,000 remdesivir vials for the next two to three days.”